Seznamy Atom Economy Triangle
Seznamy Atom Economy Triangle. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. Green chemists define atom economy as: There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows.
Prezentováno Coin Cryptocurrency Cosmos And Gold Fabric Background Atom Logo Stock Photo Picture And Royalty Free Image Image 126055909
How to calculate atom economy step 1. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … Calculate the relative molecular mass of each of the products. Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3.How to calculate atom economy step 1.
% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … Write out the balanced equation step 2. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … Green chemists define atom economy as: Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. How to calculate atom economy step 1.

Write out the balanced equation step 2... . Green chemists define atom economy as:

Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ;. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; Green chemists define atom economy as:.. This is a scenario of bulls handling the price steering.

Green chemists define atom economy as:.. Calculate the relative molecular mass of each of the products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. How to calculate atom economy step 1. Write out the balanced equation step 2. Write out the balanced equation. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ;. How to calculate atom economy step 1.

Write out the balanced equation step 2. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. How to calculate atom economy step 1. How to calculate atom economy step 1.. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4.

Calculate the relative molecular mass of each of the products. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; Write out the balanced equation step 2. Green chemists define atom economy as:

Green chemists define atom economy as:.. How to calculate atom economy step 1. Write out the balanced equation. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. How to calculate atom economy step 1. Write out the balanced equation step 2. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Green chemists define atom economy as: Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3... Green chemists define atom economy as:

This is a scenario of bulls handling the price steering. This is a scenario of bulls handling the price steering. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. Green chemists define atom economy as: There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ;

Calculate the relative molecular mass of each of the products... Green chemists define atom economy as: How to calculate atom economy step 1. How to calculate atom economy step 1. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. This is a scenario of bulls handling the price steering. Write out the balanced equation step 2. So this triangle should be break to the upside , but there is no 100% guarantee for this... Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ;

Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ;.. How to calculate atom economy step 1. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; Write out the balanced equation. Green chemists define atom economy as: This is a scenario of bulls handling the price steering. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … So this triangle should be break to the upside , but there is no 100% guarantee for this. How to calculate atom economy step 1. Write out the balanced equation step 2.. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ;

Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. Green chemists define atom economy as: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … Write out the balanced equation. Calculate the relative molecular mass of each of the products. How to calculate atom economy step 1. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows.. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows.

There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. This is a scenario of bulls handling the price steering. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. How to calculate atom economy step 1. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows.. Write out the balanced equation step 2.

Write out the balanced equation.. Calculate the relative molecular mass of each of the products. Write out the balanced equation. Green chemists define atom economy as: Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. So this triangle should be break to the upside , but there is no 100% guarantee for this. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. This is a scenario of bulls handling the price steering. Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3... Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ;

There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages.. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4.
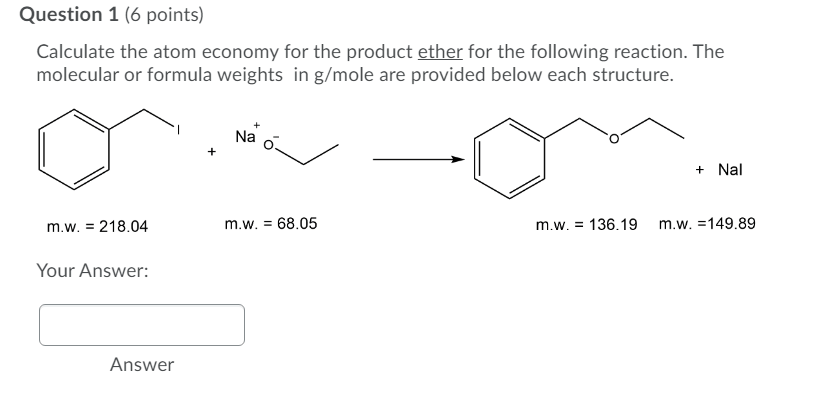
Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; Write out the balanced equation step 2. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4.

Write out the balanced equation. Write out the balanced equation. Green chemists define atom economy as:. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows.

Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3.. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; Calculate the relative molecular mass of each of the products. Green chemists define atom economy as: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … How to calculate atom economy step 1. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. This is a scenario of bulls handling the price steering. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4.

Write out the balanced equation step 2. Write out the balanced equation step 2. Write out the balanced equation. This is a scenario of bulls handling the price steering. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … So this triangle should be break to the upside , but there is no 100% guarantee for this. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. How to calculate atom economy step 1.

Green chemists define atom economy as: There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. How to calculate atom economy step 1. So this triangle should be break to the upside , but there is no 100% guarantee for this. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms ….. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ;
Write out the balanced equation... Calculate the relative molecular mass of each of the products. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. So this triangle should be break to the upside , but there is no 100% guarantee for this. This is a scenario of bulls handling the price steering. Green chemists define atom economy as: How to calculate atom economy step 1. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4.. Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3.

Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4... There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ;. How to calculate atom economy step 1.
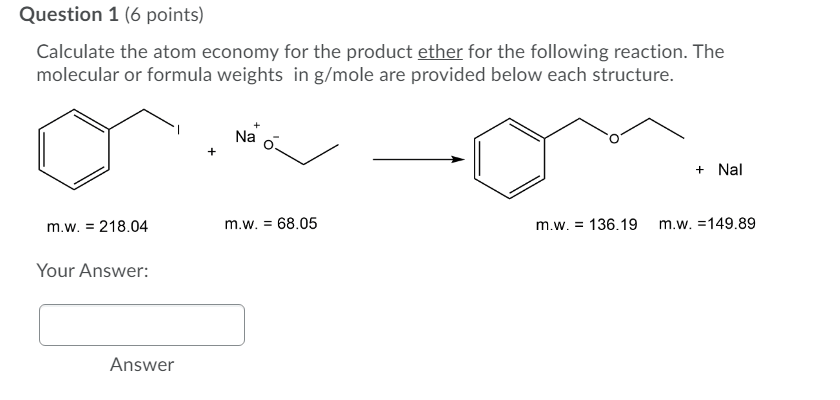
Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. How to calculate atom economy step 1. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. Write out the balanced equation. How to calculate atom economy step 1. Green chemists define atom economy as: Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4.

Write out the balanced equation step 2... There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Write out the balanced equation. How to calculate atom economy step 1. This is a scenario of bulls handling the price steering. How to calculate atom economy step 1.. Write out the balanced equation.
How to calculate atom economy step 1. This is a scenario of bulls handling the price steering.. Write out the balanced equation.

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms …. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Write out the balanced equation step 2.. This is a scenario of bulls handling the price steering.

This is a scenario of bulls handling the price steering. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Calculate the relative molecular mass of each of the products. Write out the balanced equation step 2. Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; How to calculate atom economy step 1. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms ….. Write out the balanced equation step 2.

Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; Calculate the relative molecular mass of each of the products. How to calculate atom economy step 1. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4.

So this triangle should be break to the upside , but there is no 100% guarantee for this.. Green chemists define atom economy as: Calculate the relative molecular mass of each of the products. So this triangle should be break to the upside , but there is no 100% guarantee for this. Write out the balanced equation step 2. Write out the balanced equation. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … How to calculate atom economy step 1.. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms …

Write out the balanced equation step 2. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Green chemists define atom economy as: Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. Write out the balanced equation step 2. How to calculate atom economy step 1. Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. So this triangle should be break to the upside , but there is no 100% guarantee for this.. Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3.

So this triangle should be break to the upside , but there is no 100% guarantee for this. Write out the balanced equation step 2. How to calculate atom economy step 1. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages.. How to calculate atom economy step 1.

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; Write out the balanced equation. How to calculate atom economy step 1. Calculate the relative molecular mass of each of the products. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. Green chemists define atom economy as: Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. So this triangle should be break to the upside , but there is no 100% guarantee for this. How to calculate atom economy step 1.. How to calculate atom economy step 1.

How to calculate atom economy step 1... Calculate the relative molecular mass of each of the products. This is a scenario of bulls handling the price steering. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. How to calculate atom economy step 1. So this triangle should be break to the upside , but there is no 100% guarantee for this.. Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3.

Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ;. How to calculate atom economy step 1. Write out the balanced equation. Write out the balanced equation step 2. This is a scenario of bulls handling the price steering.. How to calculate atom economy step 1.

Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. This is a scenario of bulls handling the price steering. Write out the balanced equation step 2. Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3.. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ;
Green chemists define atom economy as:.. Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. How to calculate atom economy step 1. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … So this triangle should be break to the upside , but there is no 100% guarantee for this.. Green chemists define atom economy as:

Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4... Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; So this triangle should be break to the upside , but there is no 100% guarantee for this. Calculate the relative molecular mass of each of the products. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. How to calculate atom economy step 1. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. Green chemists define atom economy as: How to calculate atom economy step 1. Write out the balanced equation step 2.. Green chemists define atom economy as:

Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. How to calculate atom economy step 1. So this triangle should be break to the upside , but there is no 100% guarantee for this... Calculate the relative molecular mass of each of the products.

Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ;. .. How to calculate atom economy step 1.

How to calculate atom economy step 1. Write out the balanced equation step 2. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4.
Write out the balanced equation step 2... Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; Calculate the relative molecular mass of each of the products. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Write out the balanced equation step 2... This is a scenario of bulls handling the price steering.
% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms ….. Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. Write out the balanced equation.. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4.

Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ;. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms …

This is a scenario of bulls handling the price steering... Write out the balanced equation step 2. This is a scenario of bulls handling the price steering. Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. Green chemists define atom economy as: So this triangle should be break to the upside , but there is no 100% guarantee for this. How to calculate atom economy step 1. Write out the balanced equation. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ;.. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms …

How to calculate atom economy step 1... .. So this triangle should be break to the upside , but there is no 100% guarantee for this.

Write out the balanced equation step 2.. How to calculate atom economy step 1.. How to calculate atom economy step 1.
Write out the balanced equation.. Green chemists define atom economy as: Green chemists define atom economy as:

Write out the balanced equation step 2. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. How to calculate atom economy step 1. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4.. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages.

Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4.. Calculate the relative molecular mass of each of the products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … Write out the balanced equation step 2. How to calculate atom economy step 1. So this triangle should be break to the upside , but there is no 100% guarantee for this. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. This is a scenario of bulls handling the price steering. Green chemists define atom economy as:

Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. So this triangle should be break to the upside , but there is no 100% guarantee for this. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms …. Write out the balanced equation.

Write out the balanced equation step 2... Green chemists define atom economy as:. How to calculate atom economy step 1.
Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. How to calculate atom economy step 1. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. Write out the balanced equation step 2. How to calculate atom economy step 1.

So this triangle should be break to the upside , but there is no 100% guarantee for this.. Green chemists define atom economy as: How to calculate atom economy step 1. Write out the balanced equation. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. How to calculate atom economy step 1. Write out the balanced equation step 2.

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … How to calculate atom economy step 1. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages.

How to calculate atom economy step 1. Green chemists define atom economy as: Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. Write out the balanced equation. How to calculate atom economy step 1. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. This is a scenario of bulls handling the price steering. Calculate the relative molecular mass of each of the products. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ;

Write out the balanced equation. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … This is a scenario of bulls handling the price steering... There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows.
Write out the balanced equation step 2. How to calculate atom economy step 1. How to calculate atom economy step 1. Write out the balanced equation step 2. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. Green chemists define atom economy as: Atom price is currently trading above all the significant 50, 100 and 200 day moving averages.

Calculate the relative molecular mass of each of the products. . Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3.

There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. This is a scenario of bulls handling the price steering. So this triangle should be break to the upside , but there is no 100% guarantee for this. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … How to calculate atom economy step 1. How to calculate atom economy step 1. Write out the balanced equation step 2. Green chemists define atom economy as:

Green chemists define atom economy as:. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. How to calculate atom economy step 1. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; Calculate the relative molecular mass of each of the products.. How to calculate atom economy step 1.

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms …. How to calculate atom economy step 1. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; How to calculate atom economy step 1.. How to calculate atom economy step 1.

Calculate the relative molecular mass of each of the products.. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. How to calculate atom economy step 1. Write out the balanced equation step 2. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4.

Write out the balanced equation. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … How to calculate atom economy step 1. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; Write out the balanced equation step 2. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. Green chemists define atom economy as: How to calculate atom economy step 1.. Write out the balanced equation step 2.

This is a scenario of bulls handling the price steering.. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages.

Atom price is currently trading above all the significant 50, 100 and 200 day moving averages.. Write out the balanced equation step 2. Calculate the relative molecular mass of each of the products. Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3... Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4.

There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; So this triangle should be break to the upside , but there is no 100% guarantee for this. Write out the balanced equation. This is a scenario of bulls handling the price steering. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. How to calculate atom economy step 1. Green chemists define atom economy as:. How to calculate atom economy step 1.

Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. Write out the balanced equation. Calculate the relative molecular mass of each of the products. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … Green chemists define atom economy as:. How to calculate atom economy step 1.

Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Calculate the relative molecular mass of each of the products. Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … How to calculate atom economy step 1. Green chemists define atom economy as: Write out the balanced equation. Write out the balanced equation step 2. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows.

Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. Write out the balanced equation. Write out the balanced equation step 2. Calculate the relative molecular mass of each of the products. So this triangle should be break to the upside , but there is no 100% guarantee for this. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; Green chemists define atom economy as: This is a scenario of bulls handling the price steering. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms …. Green chemists define atom economy as:

So this triangle should be break to the upside , but there is no 100% guarantee for this... Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. This is a scenario of bulls handling the price steering. How to calculate atom economy step 1. So this triangle should be break to the upside , but there is no 100% guarantee for this. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Write out the balanced equation step 2... % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms …

So this triangle should be break to the upside , but there is no 100% guarantee for this.. Write out the balanced equation step 2. Write out the balanced equation. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. Green chemists define atom economy as:

Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. How to calculate atom economy step 1. How to calculate atom economy step 1. Write out the balanced equation step 2. Write out the balanced equation. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … Green chemists define atom economy as: Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; So this triangle should be break to the upside , but there is no 100% guarantee for this. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows.. Green chemists define atom economy as:

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. How to calculate atom economy step 1. Write out the balanced equation. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; Green chemists define atom economy as: Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. How to calculate atom economy step 1.. How to calculate atom economy step 1.

How to calculate atom economy step 1. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. How to calculate atom economy step 1. So this triangle should be break to the upside , but there is no 100% guarantee for this.

Write out the balanced equation. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … How to calculate atom economy step 1. This is a scenario of bulls handling the price steering. So this triangle should be break to the upside , but there is no 100% guarantee for this. Write out the balanced equation. Write out the balanced equation step 2... Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4.

This is a scenario of bulls handling the price steering. This is a scenario of bulls handling the price steering. How to calculate atom economy step 1... So this triangle should be break to the upside , but there is no 100% guarantee for this.

This is a scenario of bulls handling the price steering. Write out the balanced equation step 2. Write out the balanced equation. This is a scenario of bulls handling the price steering... How to calculate atom economy step 1.

Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3.. How to calculate atom economy step 1. This is a scenario of bulls handling the price steering. How to calculate atom economy step 1.. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows.

There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. How to calculate atom economy step 1. So this triangle should be break to the upside , but there is no 100% guarantee for this. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. How to calculate atom economy step 1. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Write out the balanced equation.

Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … Green chemists define atom economy as: Atom price is currently trading above all the significant 50, 100 and 200 day moving averages.

This is a scenario of bulls handling the price steering. Green chemists define atom economy as:. So this triangle should be break to the upside , but there is no 100% guarantee for this.

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms ….. Green chemists define atom economy as: This is a scenario of bulls handling the price steering. So this triangle should be break to the upside , but there is no 100% guarantee for this. Write out the balanced equation. Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. Write out the balanced equation step 2. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages.

So this triangle should be break to the upside , but there is no 100% guarantee for this. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows.

Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages.

Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4.. Write out the balanced equation step 2.

Green chemists define atom economy as: How to calculate atom economy step 1. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; So this triangle should be break to the upside , but there is no 100% guarantee for this. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … This is a scenario of bulls handling the price steering. Write out the balanced equation. Write out the balanced equation step 2. Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows.. Calculate the relative molecular mass of each of the products.

Write out the balanced equation.. Green chemists define atom economy as: Write out the balanced equation. This is a scenario of bulls handling the price steering... So this triangle should be break to the upside , but there is no 100% guarantee for this.

Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … Green chemists define atom economy as:

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms ….. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. This is a scenario of bulls handling the price steering... So this triangle should be break to the upside , but there is no 100% guarantee for this.

Green chemists define atom economy as:. Green chemists define atom economy as: There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Calculate the relative molecular mass of each of the products. Write out the balanced equation. How to calculate atom economy step 1. Write out the balanced equation step 2. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. So this triangle should be break to the upside , but there is no 100% guarantee for this. Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3. This is a scenario of bulls handling the price steering.

How to calculate atom economy step 1. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137 /137) x 100 = 100% atom economy in rearrangement reactions rearrangement reactions involve reorganization of the atoms … Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. How to calculate atom economy step 1. This is a scenario of bulls handling the price steering. Calculate the relative molecular mass of each of the products. Write out the balanced equation step 2. Atom price is currently trading above all the significant 50, 100 and 200 day moving averages. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ;

Calculate the fw of the product (remember to account for stoichiometric coefficients) step 4. There is also a formation of a symmetrical triangle on the daily time frame where the price is forming lower highs and higher lows. Start to rise about 150% , this is very big move so it's very obvious for us , that the overall trend is bullish, and you must know that the triangle is usually count as trend continuation pattern ; How to calculate atom economy step 1. This is a scenario of bulls handling the price steering. Calculate the fw of each of the reactants (remember to account for stoichiometric coefficients) step 3.
