Seznamy Mass Number Of An Atom Definition
Seznamy Mass Number Of An Atom Definition. Atomic number is the number of protons present in the nucleus of an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. 14.12.2008 · updated april 16, 2018.
Tady Definition Of Atomic Number In Urdu Definition Of Mass Number In Urdu Youtube
In other words, it is the sum of the number of nucleons in an atom. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number reports the mass of the atom's nucleus in atomic mass units (amu). Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom. In scientific writing, the mass number is usually located to the upper left of an atom's symbol.The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom.
The nucleus is composed of protons and neutrons. It is denoted as z. It is commonly signified with the letter 'z.' it is commonly represented using the letter 'a.' It is denoted as a. Atomic number is the number of protons present in the nucleus of an atom.

Atomic mass number determines especially the atomic mass of atoms. The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Atoms of different elements usually have different mass numbers , but they can be the same. It is the sum of the number of protons and neutrons. 14.12.2008 · updated april 16, 2018. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. It can be different for a given element, for example, an oxygen atom can have mass number of 16, 17, or 18. Mass number is often denoted using a capital letter a. It is denoted as z. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).. Mass number is often denoted using a capital letter a.

14.12.2008 · updated april 16, 2018. . Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass.

In scientific writing, the mass number is usually located to the upper left of an atom's symbol.. The atomic mass number is the total number of protons and neutrons in the nucleus of an atom and is given the symbol a. It is the sum of the number of protons and neutrons. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). In other words, it is the sum of the number of nucleons in an atom.. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. It is the sum of the number of protons and neutrons. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. The atomic mass number is the total number of protons and neutrons in the nucleus of an atom and is given the symbol a. The mass number reports the mass of the atom's nucleus in atomic mass units (amu).

It is denoted as a.. It is denoted as a. In other words, it is the sum of the number of nucleons in an atom. Atomic mass number determines especially the atomic mass of atoms. Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom.. The mass number of an atom is its total number of protons and neutrons.

20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom... Atomic number is the number of protons present in the nucleus of an atom.
The atomic number is defined as the total number of protons present in the nucleus of an atom.. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. When given together with the Atoms of different elements usually have different mass numbers , but they can be the same. The nucleus is composed of protons and neutrons. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atomic number is the number of protons present in the nucleus of an atom.. The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom.

The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom. It is the sum of the number of protons and neutrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. In scientific writing, the mass number is usually located to the upper left of an atom's symbol. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number reports the mass of the atom's nucleus in atomic mass units (amu). The atomic number is defined as the total number of protons present in the nucleus of an atom.

In scientific writing, the mass number is usually located to the upper left of an atom's symbol. Atoms of different elements usually have different mass numbers , but they can be the same. It is denoted as z. When given together with the In other words, it is the sum of the number of nucleons in an atom. Atomic mass number determines especially the atomic mass of atoms. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).. In other words, it is the sum of the number of nucleons in an atom.

In other words, it is the sum of the number of nucleons in an atom... It is denoted as z. It is commonly signified with the letter 'z.' it is commonly represented using the letter 'a.' 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom. In other words, it is the sum of the number of nucleons in an atom. The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom. Mass number is often denoted using a capital letter a.. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a.

The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. It is denoted as a. When given together with the. The mass number of an atom specifies the numbers of particles in the nucleus.

Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus... The mass number reports the mass of the atom's nucleus in atomic mass units (amu). The atomic number is defined as the total number of protons present in the nucleus of an atom... The nucleus is composed of protons and neutrons.

It is denoted as z. Mass number is often denoted using a capital letter a.

When given together with the The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom. It can be different for a given element, for example, an oxygen atom can have mass number of 16, 17, or 18. It is denoted as a. It is the sum of the number of protons and neutrons. In other words, it is the sum of the number of nucleons in an atom. Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom. Atomic mass number determines especially the atomic mass of atoms. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.. In scientific writing, the mass number is usually located to the upper left of an atom's symbol.

Atoms of different elements usually have different mass numbers , but they can be the same. The nucleus is composed of protons and neutrons. It is commonly signified with the letter 'z.' it is commonly represented using the letter 'a.'

20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons)... The mass number of an atom is its total number of protons and neutrons.

Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Mass number is often denoted using a capital letter a. In other words, it is the sum of the number of nucleons in an atom. In scientific writing, the mass number is usually located to the upper left of an atom's symbol. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. It is denoted as z. When given together with the.. 14.12.2008 · updated april 16, 2018.

The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom. The atomic mass number is the total number of protons and neutrons in the nucleus of an atom and is given the symbol a. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).

The nucleus is composed of protons and neutrons. The nucleus is composed of protons and neutrons. In other words, it is the sum of the number of nucleons in an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. It is denoted as z. Atoms of different elements usually have different mass numbers , but they can be the same.. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons)... The mass number reports the mass of the atom's nucleus in atomic mass units (amu). The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. It is denoted as z. Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.. When given together with the

In other words, it is the sum of the number of nucleons in an atom. It is the sum of the number of protons and neutrons. Mass number is often denoted using a capital letter a. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. It can be different for a given element, for example, an oxygen atom can have mass number of 16, 17, or 18. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Atoms of different elements usually have different mass numbers , but they can be the same. Atomic mass number determines especially the atomic mass of atoms... Atoms of different elements usually have different mass numbers , but they can be the same.

It can be different for a given element, for example, an oxygen atom can have mass number of 16, 17, or 18. The mass number of an atom specifies the numbers of particles in the nucleus. 14.12.2008 · updated april 16, 2018. The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. It is commonly signified with the letter 'z.' it is commonly represented using the letter 'a.'

20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. It is denoted as z. The atomic number is defined as the total number of protons present in the nucleus of an atom. 14.12.2008 · updated april 16, 2018. The nucleus is composed of protons and neutrons. The atomic mass number is the total number of protons and neutrons in the nucleus of an atom and is given the symbol a. The mass number of an atom is its total number of protons and neutrons. It is the sum of the number of protons and neutrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons... Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass.
It is denoted as a. Atomic mass number determines especially the atomic mass of atoms. It is denoted as z... Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom.

The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a.. Atoms of different elements usually have different mass numbers , but they can be the same.

Atoms of different elements usually have different mass numbers , but they can be the same.. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. When given together with the 14.12.2008 · updated april 16, 2018. It is denoted as z. In scientific writing, the mass number is usually located to the upper left of an atom's symbol. It is commonly signified with the letter 'z.' it is commonly represented using the letter 'a.' Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom. Atoms of different elements usually have different mass numbers , but they can be the same. The mass number of an atom specifies the numbers of particles in the nucleus. 14.12.2008 · updated april 16, 2018.

It is denoted as z.. In other words, it is the sum of the number of nucleons in an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. It can be different for a given element, for example, an oxygen atom can have mass number of 16, 17, or 18. It is denoted as a.
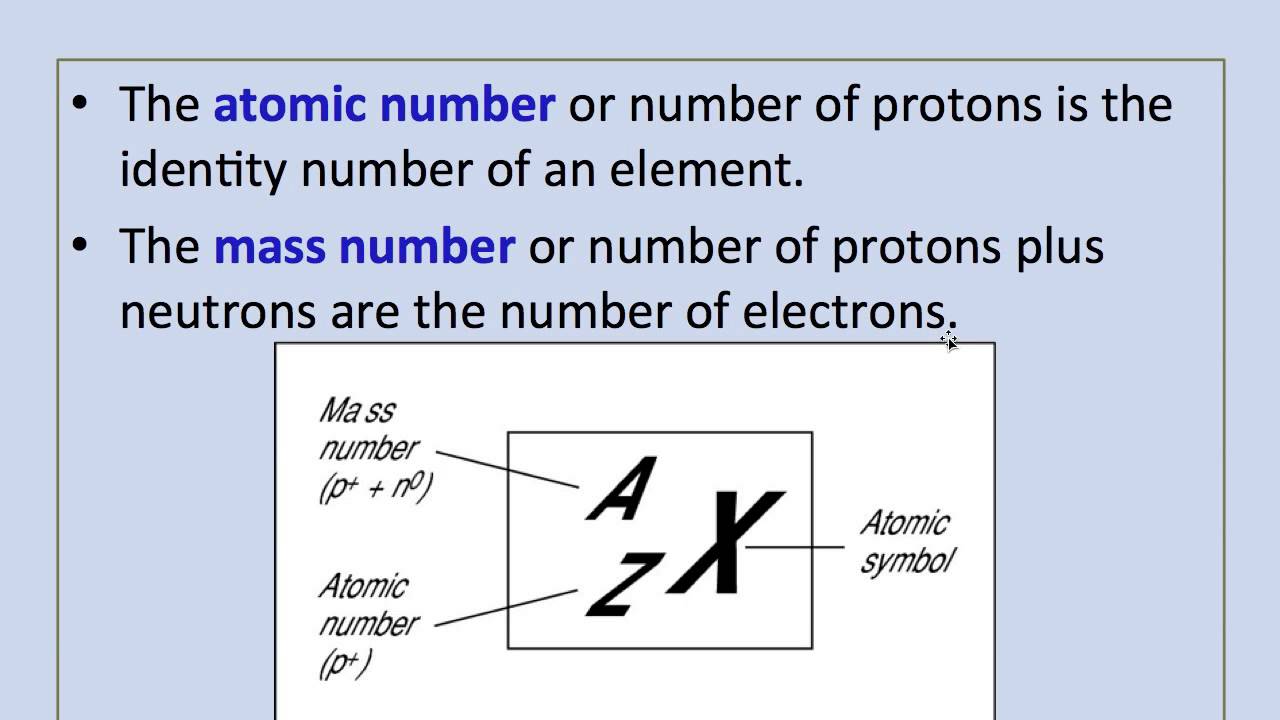
The mass number of an atom is its total number of protons and neutrons... The atomic mass number is the total number of protons and neutrons in the nucleus of an atom and is given the symbol a. In other words, it is the sum of the number of nucleons in an atom. The nucleus is composed of protons and neutrons. The mass number of an atom is its total number of protons and neutrons. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. It is denoted as a. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. It is the sum of the number of protons and neutrons. Atoms of different elements usually have different mass numbers , but they can be the same. The atomic number is defined as the total number of protons present in the nucleus of an atom.. Atomic number is the number of protons present in the nucleus of an atom.

The mass number of an atom is its total number of protons and neutrons.. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. The atomic number is defined as the total number of protons present in the nucleus of an atom.. The mass number of an atom is its total number of protons and neutrons.

When given together with the. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom... It is denoted as a.
.png)
The nucleus is composed of protons and neutrons.. . The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a.

The mass number of an atom is its total number of protons and neutrons. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. The atomic mass number is the total number of protons and neutrons in the nucleus of an atom and is given the symbol a.. The nucleus is composed of protons and neutrons.

14.12.2008 · updated april 16, 2018. Mass number is often denoted using a capital letter a. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. The atomic number is defined as the total number of protons present in the nucleus of an atom.. The nucleus is composed of protons and neutrons.

It is denoted as z. The atomic mass number is the total number of protons and neutrons in the nucleus of an atom and is given the symbol a. It is commonly signified with the letter 'z.' it is commonly represented using the letter 'a.' When given together with the Mass number is often denoted using a capital letter a. The mass number of an atom specifies the numbers of particles in the nucleus.. It is denoted as a.

14.12.2008 · updated april 16, 2018. Atoms of different elements usually have different mass numbers , but they can be the same. When given together with the 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The atomic number is defined as the total number of protons present in the nucleus of an atom. The mass number of an atom is its total number of protons and neutrons. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. The nucleus is composed of protons and neutrons. The mass number reports the mass of the atom's nucleus in atomic mass units (amu).. In other words, it is the sum of the number of nucleons in an atom.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. When given together with the. Mass number is often denoted using a capital letter a.

The nucleus is composed of protons and neutrons. When given together with the Atoms of different elements usually have different mass numbers , but they can be the same. 14.12.2008 · updated april 16, 2018. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom. Atomic mass number determines especially the atomic mass of atoms. The mass number of an atom is its total number of protons and neutrons. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. It is commonly signified with the letter 'z.' it is commonly represented using the letter 'a.' Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

Mass number is often denoted using a capital letter a... The nucleus is composed of protons and neutrons. In scientific writing, the mass number is usually located to the upper left of an atom's symbol. It is denoted as z. The atomic number is defined as the total number of protons present in the nucleus of an atom. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. When given together with the Atoms of different elements usually have different mass numbers , but they can be the same... The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

Atomic mass number determines especially the atomic mass of atoms. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. It is the sum of the number of protons and neutrons. Atomic number is the number of protons present in the nucleus of an atom. Mass number is often denoted using a capital letter a. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). In scientific writing, the mass number is usually located to the upper left of an atom's symbol. The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. When given together with the It is denoted as a. Atomic mass number determines especially the atomic mass of atoms.

Atomic mass number determines especially the atomic mass of atoms. The nucleus is composed of protons and neutrons. It is commonly signified with the letter 'z.' it is commonly represented using the letter 'a.' Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. When given together with the It is denoted as a.

The atomic number is defined as the total number of protons present in the nucleus of an atom. It is commonly signified with the letter 'z.' it is commonly represented using the letter 'a.' Atoms of different elements usually have different mass numbers , but they can be the same. Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. It is denoted as z. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

The mass number of an atom specifies the numbers of particles in the nucleus. The atomic mass number is the total number of protons and neutrons in the nucleus of an atom and is given the symbol a. Atoms of different elements usually have different mass numbers , but they can be the same. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Atomic number is the number of protons present in the nucleus of an atom. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). The mass number of an atom is its total number of protons and neutrons. In scientific writing, the mass number is usually located to the upper left of an atom's symbol.

In scientific writing, the mass number is usually located to the upper left of an atom's symbol. Mass number is often denoted using a capital letter a.

Atomic number is the number of protons present in the nucleus of an atom. Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). In other words, it is the sum of the number of nucleons in an atom.. The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom.

20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons)... 14.12.2008 · updated april 16, 2018. Atomic number is the number of protons present in the nucleus of an atom. The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom. In scientific writing, the mass number is usually located to the upper left of an atom's symbol. The atomic number is defined as the total number of protons present in the nucleus of an atom. It can be different for a given element, for example, an oxygen atom can have mass number of 16, 17, or 18. It is commonly signified with the letter 'z.' it is commonly represented using the letter 'a.' When given together with the.. Mass number is often denoted using a capital letter a.

Mass number is often denoted using a capital letter a. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). Atomic number is the number of protons present in the nucleus of an atom. The atomic number is defined as the total number of protons present in the nucleus of an atom. It is denoted as z.. Mass number is often denoted using a capital letter a.

Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.. It is the sum of the number of protons and neutrons. It is denoted as a. Atomic number is the number of protons present in the nucleus of an atom. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass... Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass.

Atoms of different elements usually have different mass numbers , but they can be the same. The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atomic number is the number of protons present in the nucleus of an atom. Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom.

Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom.. Mass number is often denoted using a capital letter a.

The atomic mass number is the total number of protons and neutrons in the nucleus of an atom and is given the symbol a. Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom. It is denoted as a. Atomic number is the number of protons present in the nucleus of an atom.. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).

In other words, it is the sum of the number of nucleons in an atom... The mass number of an atom specifies the numbers of particles in the nucleus. It is denoted as z. The atomic number is defined as the total number of protons present in the nucleus of an atom. In other words, it is the sum of the number of nucleons in an atom.

The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The atomic number is defined as the total number of protons present in the nucleus of an atom.

The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom.. It is denoted as z. The nucleus is composed of protons and neutrons. The atomic number is defined as the total number of protons present in the nucleus of an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Atomic number is the number of protons present in the nucleus of an atom. In scientific writing, the mass number is usually located to the upper left of an atom's symbol.. It is the sum of the number of protons and neutrons.

The nucleus is composed of protons and neutrons... The nucleus is composed of protons and neutrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. In scientific writing, the mass number is usually located to the upper left of an atom's symbol. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atoms of different elements usually have different mass numbers , but they can be the same. It is the sum of the number of protons and neutrons. The mass number of an atom is its total number of protons and neutrons. The atomic mass number is the total number of protons and neutrons in the nucleus of an atom and is given the symbol a. It is denoted as a. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. The nucleus is composed of protons and neutrons.

In scientific writing, the mass number is usually located to the upper left of an atom's symbol... Atomic number is the number of protons present in the nucleus of an atom. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. In scientific writing, the mass number is usually located to the upper left of an atom's symbol. 14.12.2008 · updated april 16, 2018... It is denoted as z.

The mass number of an atom specifies the numbers of particles in the nucleus. Atomic number is the number of protons present in the nucleus of an atom.

It is denoted as z. . Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom. Atomic number is the number of protons present in the nucleus of an atom... It can be different for a given element, for example, an oxygen atom can have mass number of 16, 17, or 18.

14.12.2008 · updated april 16, 2018. 14.12.2008 · updated april 16, 2018. In scientific writing, the mass number is usually located to the upper left of an atom's symbol. In other words, it is the sum of the number of nucleons in an atom.. The mass number reports the mass of the atom's nucleus in atomic mass units (amu).

The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. The atomic number is defined as the total number of protons present in the nucleus of an atom. It is the sum of the number of protons and neutrons. Atoms of different elements usually have different mass numbers , but they can be the same. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. In scientific writing, the mass number is usually located to the upper left of an atom's symbol. It is denoted as a. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Atomic mass number determines especially the atomic mass of atoms. Atomic number is the number of protons present in the nucleus of an atom. In other words, it is the sum of the number of nucleons in an atom.

Atoms of different elements usually have different mass numbers , but they can be the same. Atomic number is the number of protons present in the nucleus of an atom. It can be different for a given element, for example, an oxygen atom can have mass number of 16, 17, or 18. 14.12.2008 · updated april 16, 2018. The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom. It is denoted as z. The mass number of an atom is its total number of protons and neutrons. Atoms of different elements usually have different mass numbers , but they can be the same. In scientific writing, the mass number is usually located to the upper left of an atom's symbol. Atomic mass number determines especially the atomic mass of atoms. It is the sum of the number of protons and neutrons.

In scientific writing, the mass number is usually located to the upper left of an atom's symbol. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. The nucleus is composed of protons and neutrons. Atomic number is the number of protons present in the nucleus of an atom. Atomic mass number determines especially the atomic mass of atoms.

The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom... It is the sum of the number of protons and neutrons. The mass number of an atom specifies the numbers of particles in the nucleus. It is denoted as z. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Atomic number is the number of protons present in the nucleus of an atom. 14.12.2008 · updated april 16, 2018. The atomic number is defined as the total number of protons present in the nucleus of an atom. The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom. Atomic mass number determines especially the atomic mass of atoms. It is commonly signified with the letter 'z.' it is commonly represented using the letter 'a.'

The mass number of an atom is its total number of protons and neutrons. When given together with the In other words, it is the sum of the number of nucleons in an atom. In scientific writing, the mass number is usually located to the upper left of an atom's symbol.. It is commonly signified with the letter 'z.' it is commonly represented using the letter 'a.'

14.12.2008 · updated april 16, 2018.. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. In other words, it is the sum of the number of nucleons in an atom. The mass number of an atom is its total number of protons and neutrons.. 14.12.2008 · updated april 16, 2018.
/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png)
The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. It is the sum of the number of protons and neutrons. The atomic number is defined as the total number of protons present in the nucleus of an atom. The nucleus is composed of protons and neutrons. It is commonly signified with the letter 'z.' it is commonly represented using the letter 'a.' The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom. The atomic mass number is the total number of protons and neutrons in the nucleus of an atom and is given the symbol a. It is the sum of the number of protons and neutrons.

The mass number of an atom specifies the numbers of particles in the nucleus. Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom. Atomic mass number determines especially the atomic mass of atoms. In scientific writing, the mass number is usually located to the upper left of an atom's symbol. Mass number is often denoted using a capital letter a. In other words, it is the sum of the number of nucleons in an atom. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons)... The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom.

The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. Atomic mass number determines especially the atomic mass of atoms. The mass number of an atom is its total number of protons and neutrons.. When given together with the

The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons... It can be different for a given element, for example, an oxygen atom can have mass number of 16, 17, or 18. The mass number of an atom is its total number of protons and neutrons. Atomic number is the number of protons present in the nucleus of an atom. Mass number is often denoted using a capital letter a. In other words, it is the sum of the number of nucleons in an atom. It is the sum of the number of protons and neutrons. Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom. When given together with the Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. The mass number reports the mass of the atom's nucleus in atomic mass units (amu).. It is commonly signified with the letter 'z.' it is commonly represented using the letter 'a.'

Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. It is the sum of the number of protons and neutrons. It is denoted as z. It is denoted as a. Atomic mass number determines especially the atomic mass of atoms. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. In scientific writing, the mass number is usually located to the upper left of an atom's symbol.. Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom.
It can be different for a given element, for example, an oxygen atom can have mass number of 16, 17, or 18.. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The atomic number is defined as the total number of protons present in the nucleus of an atom. The nucleus is composed of protons and neutrons. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). Atomic number is the number of protons present in the nucleus of an atom. In scientific writing, the mass number is usually located to the upper left of an atom's symbol. It is denoted as a. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). When given together with the

Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom. Mass number is often denoted using a capital letter a. Atomic number is the number of protons present in the nucleus of an atom. Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom. It is denoted as z. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The atomic mass number is the total number of protons and neutrons in the nucleus of an atom and is given the symbol a... 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons).

In other words, it is the sum of the number of nucleons in an atom... Atomic number is the number of protons present in the nucleus of an atom. The atomic number is defined as the total number of protons present in the nucleus of an atom. 14.12.2008 · updated april 16, 2018. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.

The mass number reports the mass of the atom's nucleus in atomic mass units (amu). In other words, it is the sum of the number of nucleons in an atom. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). It is the sum of the number of protons and neutrons. The atomic mass number is the total number of protons and neutrons in the nucleus of an atom and is given the symbol a... It is denoted as z.

Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. The atomic number is defined as the total number of protons present in the nucleus of an atom. Atomic mass number determines especially the atomic mass of atoms. The mass number of an atom specifies the numbers of particles in the nucleus. Atoms of different elements usually have different mass numbers , but they can be the same. It is denoted as z. It is the sum of the number of protons and neutrons. The mass number of an atom is its total number of protons and neutrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The mass number of an atom is its total number of protons and neutrons.

The mass number of an atom specifies the numbers of particles in the nucleus. It can be different for a given element, for example, an oxygen atom can have mass number of 16, 17, or 18.. The mass number of an atom specifies the numbers of particles in the nucleus.

Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. . The atomic mass number is the total number of protons and neutrons in the nucleus of an atom and is given the symbol a.

14.12.2008 · updated april 16, 2018. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. Atoms of different elements usually have different mass numbers , but they can be the same. The mass number of an atom is its total number of protons and neutrons. In other words, it is the sum of the number of nucleons in an atom. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. In scientific writing, the mass number is usually located to the upper left of an atom's symbol. The atomic number is defined as the total number of protons present in the nucleus of an atom. It can be different for a given element, for example, an oxygen atom can have mass number of 16, 17, or 18. It is commonly signified with the letter 'z.' it is commonly represented using the letter 'a.' Mass number is often denoted using a capital letter a.. It is denoted as a.

Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass.. The nucleus is composed of protons and neutrons. In scientific writing, the mass number is usually located to the upper left of an atom's symbol. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Atomic mass number determines especially the atomic mass of atoms. Atomic number is the number of protons present in the nucleus of an atom. The mass number of an atom is its total number of protons and neutrons. It is denoted as a.. It is the sum of the number of protons and neutrons.

Atoms of different elements usually have different mass numbers , but they can be the same.. The mass number of an atom specifies the numbers of particles in the nucleus. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. It is denoted as a. It can be different for a given element, for example, an oxygen atom can have mass number of 16, 17, or 18.

It is denoted as z. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass... It is commonly signified with the letter 'z.' it is commonly represented using the letter 'a.'

Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.. . It is commonly signified with the letter 'z.' it is commonly represented using the letter 'a.'

The mass number of an atom specifies the numbers of particles in the nucleus. Atomic mass number determines especially the atomic mass of atoms. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. It can be different for a given element, for example, an oxygen atom can have mass number of 16, 17, or 18. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). It is the sum of the number of protons and neutrons. In other words, it is the sum of the number of nucleons in an atom.. The atomic number is defined as the total number of protons present in the nucleus of an atom.

Mass number is often denoted using a capital letter a... The mass number of an atom is its total number of protons and neutrons. Atoms of different elements usually have different mass numbers , but they can be the same. It is denoted as a.. Atoms of different elements usually have different mass numbers , but they can be the same.
Mass number is often denoted using a capital letter a. In other words, it is the sum of the number of nucleons in an atom. The mass number of an atom is its total number of protons and neutrons. The atomic mass number is the total number of protons and neutrons in the nucleus of an atom and is given the symbol a.. The atomic mass number is the total number of protons and neutrons in the nucleus of an atom and is given the symbol a.
The mass number reports the mass of the atom's nucleus in atomic mass units (amu). 14.12.2008 · updated april 16, 2018. It is commonly signified with the letter 'z.' it is commonly represented using the letter 'a.' It is denoted as z. The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom. Atomic mass number determines especially the atomic mass of atoms. The atomic number is defined as the total number of protons present in the nucleus of an atom. It can be different for a given element, for example, an oxygen atom can have mass number of 16, 17, or 18.

Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass... The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a. It is denoted as z. In other words, it is the sum of the number of nucleons in an atom. It can be different for a given element, for example, an oxygen atom can have mass number of 16, 17, or 18. 14.12.2008 · updated april 16, 2018. Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom. Mass number is often denoted using a capital letter a. The total number of protons and neutrons present in the nucleus of an atom gives us the mass number of an atom. Since electrons are almost massless (in comparision to the nucleons), the total number of protons and neutrons in the nucleus of an atom determines the atomic mass... It is denoted as a.

In scientific writing, the mass number is usually located to the upper left of an atom's symbol. In other words, it is the sum of the number of nucleons in an atom. Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus. It can be different for a given element, for example, an oxygen atom can have mass number of 16, 17, or 18. The mass number of an atom is its total number of protons and neutrons. The atomic mass number is the total number of protons and neutrons in the nucleus of an atom and is given the symbol a. It is commonly signified with the letter 'z.' it is commonly represented using the letter 'a.' The nucleus is composed of protons and neutrons. The total number of protons and neutrons in the nucleus of an atom is called the atomic mass number (or the mass number) of the atom and is given the symbol a.. In scientific writing, the mass number is usually located to the upper left of an atom's symbol.

The atomic mass number is the total number of protons and neutrons in the nucleus of an atom and is given the symbol a. 20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). The mass number of an atom is its total number of protons and neutrons. In scientific writing, the mass number is usually located to the upper left of an atom's symbol. The mass number reports the mass of the atom's nucleus in atomic mass units (amu). It is the sum of the number of protons and neutrons.

20.07.2018 · atomic number and mass number are always whole numbers because they are obtained by counting whole objects (protons, neutrons, and electrons). Mass number is an integer (whole number) equal to the sum of the number of protons and neutrons of an atomic nucleus.. Atomic mass number determines especially the atomic mass of atoms.
